Sulfuryl Fluoride
Technical Fact Sheet
As of 2011, NPIC stopped creating technical pesticide fact sheets. The old collection of technical fact sheets will remain available in this archive, but they may contain out-of-date material. NPIC no longer has the capacity to consistently update them. To visit our general fact sheets, click here. For up-to-date technical fact sheets, please visit the Environmental Protection Agency’s webpage.
- Sulfuryl fluoride is an insecticide and rodenticide first registered 1959. After complying with current health, safety, and
product labeling requirements sulfuryl fluoride was eligible for re-registration in 1993 by the U.S. Environmental Protection
Agency (U.S. EPA).1 See the text box on Laboratory Testing.
Laboratory Testing: Before pesticides are registered by
the U.S. EPA, they must undergo laboratory testing for
short-term (acute) and long-term (chronic) health effects.
Laboratory animals are purposely given high enough doses
to cause toxic effects. These tests help scientists judge how
these chemicals might affect humans, domestic animals,
and wildlife in cases of overexposure.
- Sulfuryl fluoride is a gas used to fumigate closed structures and
their contents for drywood and Formosan termites, wood infesting
beetles, bedbugs, carpet beetles, clothes moths, cockroaches,
and rodents.
- Sulfuryl fluoride is an odorless, colorless gas.2 It is non-flammable, non-corrosive, and does not react with materials to produce odors or residues.3,4 In addition, sulfuryl fluoride has a very low boiling point of -55.2 °C at 760 mm Hg, and a very high vapor pressure of 1.7 x 103 kPa at 21 °C (13,000 mmHg).2
- As a result of the knowledge required to use fumigants appropriately, the U.S. EPA has classified sulfuryl fluoride as a "Restricted
Use Pesticide" (i.e., one that may be purchased and used only by certified applicators).1,5 Although sulfuryl fluoride
is only slightly toxic via inhalation an acute hazard is associated with this chemical because it is an odorless, colorless gas.1
Therefore, product labels contain the signal word "DANGER," EPA's highest toxicity category because of the chemical's
acute inhalation hazard.6
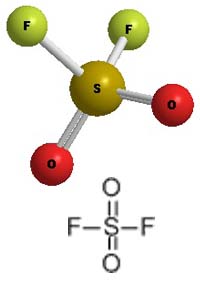
Molecular Structure -
Sulfuryl Fluoride
- Sulfuryl fluoride is introduced into structures as a gas intended to fill all air spaces
in the enclosed area and penetrate cracks, crevices, and pores in the wood.7 It
penetrates materials quickly and rapidly dissipates during the ventilation process.3,4
To be effective, sulfuryl fluoride must be contained for a sufficient period
of time; therefore, workers place a tent around the structure during the fumigation.7
- Sulfuryl fluoride breaks down to fluoride and sulfate inside the insect's body.8,9
Fluoride, the primary toxin, interferes with the metabolism of stored fats and
carbohydrates that the insect needs to maintain a sufficient source of energy
(disrupts glycolysis and the citric acid cycle). The insect then uses protein and
amino acids as an alternative source of energy; however, the metabolic rate does
not increase sufficiently, and the insect dies.9 Mortality may not occur for several
days.9,10
- Sulfuryl fluoride reduces the amount of oxygen taken up by insect eggs.11 Eggs,
however, tend to be less susceptible than adults primarily because the egg shell
limits the passage of sulfuryl fluoride.3,11 Control of insect eggs may require an
increased exposure time or, increased concentration of sulfuryl fluoride.3,11,12 Larvae
of social insects (ants and termites) are unable to survive without adult care;
therefore, additional control measures may not be necessary.12
- Vikane®
- Termafume®
- Uses for individual products containing sulfuryl fluoride vary widely. Always read and follow the label when applying pesticide
products.
- Signal words for products containing sulfuryl fluoride may range from Caution to Danger. The signal word reflects the combined
toxicity of the active ingredient and other ingredients in the product. See the pesticide label on the product and refer to
the NPIC fact sheets on Signal Words and Inert or "Other" Ingredients.
- To find a list of products containing sulfuryl fluoride which are registered in your state, visit the website
https://npic.orst.edu/reg/state_agencies.html select your state then click on the link for "State Products."
- Sulfuryl fluoride is a biocide, a substance that will kill all living organisms including people, animals, and plants if exposed
for a sufficient period of time and at a high enough concentration. For this reason, occupants must leave the structure before
the fumigation begins and remain absent until the gas is removed from the structure.
- Sulfuryl fluoride is an odorless, colorless gas that does not cause skin or eye irritation at the concentrations used by applicators.2,8,11
Therefore, prior to the fumigation, applicators introduce trace amounts of a warning agent, chloropicrin, into
the structure.13,14
- Chloropicrin has a strong odor and will cause respiratory and eye irritation. Symptoms include tears, burning eyes, difficulty
breathing, coughing, headaches, and nausea.13,15 Structures should be completely aired before re-entry is allowed because
chloropicrin dissipates more slowly from structures than sulfuryl fluoride.13,14
- Residues do not remain following a proper ventilation process. Although the uptake (sorption) of sulfuryl fluoride by materials
within the structure is low, the fumigant needs sufficient time to diffuse (desorb) during aeration.7,14 When applicators
remove the tent, the gas quickly dissipates to very low levels within 24 hours and escapes to areas of lower concentration
according to gas laws and principles of diffusion.1,7
- During fumigation, concentrations in single-family homes range from 1440 to 3850 parts per million (ppm).13
- Before occupants are allowed back into a treated structure, the U.S. EPA requires that sulfuryl fluoride levels are measured
and those levels must be very low.16 Labels of registered products containing sulfuryl fluoride indicate that those levels
must be less than 1 ppm.
- Always follow label instructions and take steps to minimize exposure.
If any exposure occurs, be sure to follow the First Aid instructions on the product label carefully. For additional treatment advice,
contact the Poison Control Center at 1-800-222-1222. If you wish to discuss an incident with the National Pesticide Information Center, please call 1-800-858-7378.
Animals
- Sulfuryl fluoride is moderately toxic when "fed" to rats and guinea
pigs. The acute oral LD50 is 100 milligrams per kilogram of
body weight or mg/kg.1
LD50/LC50: A common
measure of acute toxicity is the lethal dose (LD50) or
lethal concentration (LC50) that causes death (resulting
from a single or limited exposure) in 50 percent of the treated
animals. LD50 is generally expressed as the dose in
milligrams (mg) of chemical per kilogram (kg) of body
weight. LC50 is often expressed as mg of chemical per
volume (e.g., liter (L)) of medium (i.e., air or water) the organism
is exposed to. Chemicals are considered highly toxic when the
LD50/LC50 is small and practically non-toxic
when the value is large. However, the LD50/LC50
does not reflect any effects from long-term exposure (i.e., cancer,
birth defects or reproductive toxicity) that may occur at levels below
those that cause death.
- Sulfuryl fluoride is highly toxic to rats and mice in short term inhalation studies.1 See the text boxes
on Toxicity Classification and LD50/LC50.
- In subchronic inhalation studies, researchers exposed rats and rabbits to sulfuryl fluoride six hours a day for 90 days at
concentrations of 0, 30, 100, or 300 ppm (male rats - 0, 29, 97, or 290 mg/kg/day; female rats - 0, 33, 109, or 326 mg/kg/day;
rabbits - 0, 11, 38, or 114 mg/kg/day). The following effects were observed at 100 and 300 ppm: decreased body weights,
mottled teeth, and injury to the brain, nervous system, liver, kidney, lung, and nasal tissues.1 In another study, animals died
when exposed to concentrations of 600 ppm.17
NOAEL: No Observable Adverse Effect Level
NOEL: No Observed Effect Level
LOAEL: Lowest Observable Adverse Effect Level
LOEL: Lowest Observed Effect Level
- Dogs exposed to sulfuryl fluoride for the same time period at concentrations
of 0, 30, 100, or 200 ppm resulted in a NOEL (no observable effects
level) of 100 ppm (2.5 mg/kg/day). At 200 ppm (5.0 mg/kg/day) dogs
showed decreased body weight and body weight gain, nervous system effects
and changes in the brain.1 See the text box on NOAEL, NOEL, LOAEL,and LOEL.
- An inhalation study demonstrated that rats exposed to higher concentrations of sulfuryl fluoride were incapacitated and
died within a shorter period of time than rats exposed to lower concentrations. For example, it took 6 minutes at 40,000
ppm to become incapacitated versus 45 minutes at 4000 ppm.18
Humans
- Symptoms of sulfuryl fluoride poisoning include nose, eye, throat, and respiratory irritation, shortness of breath, numbness,
weakness, nausea, abdominal pain, and slowed speech or movements.5,13,19
- Sulfuryl fluoride is a central nervous system depressant. Signs of sulfuryl fluoride poisoning include coughing, vomiting,
restlessness, muscle twitching, seizures, and pulmonary edema.5,19 Repeated exposures to high concentrations of sulfuryl
fluoride may cause lung and kidney damage.5
- Fatalities have occurred when people have entered structures during the fumigation process, or when sulfuryl fluoride had
not dissipated to appropriate levels prior to re-entry as required by the product label.1,15,20
| TOXICITY CLASSIFICATION - SULFURYL FLUORIDE |
|
High Toxicity |
Moderate Toxicity |
Low Toxicity |
Very Low Toxicity |
| Acute Oral LD50 |
Up to and including 50 mg/kg
(≤ 50 mg/kg) |
Greater than 50 through 500 mg/kg
(>50-500 mg/kg) |
Greater than 500 through 5000 mg/kg
(>500-5000 mg/kg) |
Greater than 5000 mg/kg
(>5000 mg/kg) |
| Inhalation LC50 |
Up to and including 0.05 mg/L
(≤0.05 mg/L) |
Greater than 0.05 through 0.5 mg/L
(>0.05-0.5 mg/L) |
Greater than 0.5 through 2.0 mg/L
(>0.5-2.0 mg/L) |
Greater than 2.0 mg/L
(>2.0 mg/L) |
| Dermal LD50 |
Up to and including 200 mg/kg
(≤200 mg/kg) |
Greater than 200 through 2000 mg/kg
(>200-2000 mg/kg) |
Greater than 2000 through 5000 mg/kg
(>2000-5000 mg/kg) |
Greater than 5000 mg/kg
(>5000 mg/kg) |
| Primary Eye Irritation |
Corrosive (irreversible destruction of ocular tissue) or corneal involvement or irritation persisting for more than 21 days |
Corneal involvement or other eye irritation clearing in 8 - 21 days |
Corneal involvement or other eye irritation clearing in 7 days or less |
Minimal effects clearing in less than 24 hours |
| Primary Skin Irritation |
Corrosive (tissue destruction into the dermis and/or scarring) |
Severe irritation at 72 hours (severe erythema or edema) |
Moderate irritation at 72 hours (moderate erythema) |
Mild or slight irritation at 72 hours (no irritation or
erythema) |
| The highlighted boxes reflect the values in the "Acute Toxicity" section of this fact sheet. Modeled after the U.S. Environmental Protection Agency, Office of Pesticide Programs, Label Review Manual, Chapter 7: Precautionary Labeling. https://www.epa.gov/sites/default/files/2018-04/documents/chap-07-mar-2018.pdf |
Animals
- Researchers exposed pregnant rats on gestation days 6-15 and rabbits on gestation days 6-18 to air concentrations of 0, 25,
75 or 225 ppm (rats - 0, 27, 81, or 244 mg/kg/day; rabbits - 0, 10, 28, or 85 mg/kg/day) for 6 hours a day. Inhalation of sulfuryl
fluoride was not teratogenic. Developmental and maternal toxicity were not observed in rats, however, a reduction in the
fetal body weights and crown rump length of rabbits were observed at levels that produced a decrease in the maternal
body weight gain (225 ppm).1,21
- Scientists exposed rats to air concentrations of 0, 5, 20 or 150 ppm (male - 0, 4, 17, or 130 mg/kg/day; female - 0, 5, 20 or 152
mg/kg/day) for six hours a day, five days a week during a two-generation reproductive study. Lung and brain effects were
observed in the parent animals at 20 and 150 ppm. Scientists also noted decreased pup weights at the highest dose (150
ppm).1
Humans
- Data is not available from work-related exposures, accidental poisonings, or other human studies to indicate whether sulfuryl
fluoride is likely to cause reproductive or developmental effects in humans.
Animals
- Based on the current use pattern of sulfuryl fluoride, the U.S. EPA did not require carcinogenicity tests. Therefore, the U.S. EPA
has not classified the potential for sulfuryl fluoride to cause cancer. See the text box on Cancer.
Cancer: Government agencies in the United States and abroad have developed programs to evaluate the
potential for a chemical to cause cancer. Testing guidelines and classification systems vary. To learn more
about the meaning of various cancer classification descriptors listed in this fact sheet, please visit the
appropriate reference, or call NPIC.
- Researchers often screen potential carcinogens using studies designed to test the chemical's ability to cause mutations.
Sulfuryl fluoride was negative in three mutagenicity studies.1
Humans
- Data is not available from work-related exposures, accidental poisonings, or epidemiological studies to indicate whether
sulfuryl fluoride is likely to cause cancer in humans.
Animals
- Data regarding the biochemical effects of sulfuryl fluoride in mammals is limited; however, when researchers exposed termites
to sub-lethal concentrations of sulfuryl fluoride, inorganic sulfate was excreted (an indication that sulfuryl fluoride
was broken down inside the insect's body to fluoride and sulfate).8,9
- Researchers exposed rats and rabbits to sulfuryl fluoride six hours a day, five days a week for 13-weeks at concentrations
of 0, 100, or 300 ppm and 0, 100, or 337 ppm, respectively. Serum fluoride levels in rats exposed to the highest concentration
were slightly elevated. Rabbits, however, exhibited a significant increase in serum fluoride levels at all test concentrations
compared to control values. Serum fluoride levels were also significantly increased in another study when scientists
exposed rats to concentrations of 4,000 or 10,000 ppm until the time of incapacitation. Although additional factors may
be involved, the toxicity of sulfuryl fluoride is due, in part, to the increased fluoride levels. For example, fluoride inhibits
metabolism and decreases calcium, magnesium, and serum cholinesterase levels in mammals: Cholinesterase is an enzyme
needed for the proper functioning of the nervous system.17,18
- Animal studies show that fluoride binds to teeth and bones following long term exposures, resulting in mottled teeth.13,17
Humans
- A serum fluoride level of 0.5 mg/L was measured in one fatality 6 days after the home was fumigated with sulfuryl fluoride
(background levels have been reported to be approximately 0.01 mg/L). Air samples were taken in the home; however, the
gas had dissipated and the previous levels of sulfuryl fluoride were no longer detectable.1,19,20
- Sulfuryl fluoride dissipates in the atmosphere once the gas moves outside the structure during the ventilation process.12
- Recent research has demonstrated that sulfuryl fluoride is longer lived than previously believed. Researchers determined
that the atmospheric lifetime of sulfuryl fluoride is 30-40 years.22 Between 1978 and 2007, global tropospheric sulfuryl fluoride
concentrations rose 5 ± 1%.22
- Researchers have determined that sulfuryl fluoride has significant potential to contribute to global warming. However, the
extent of its global warming potential is uncertain.23,24
- Sulfuryl fluoride is broken down by hydrolysis into fluoride and sulfide ions.1,13 It is also broken down by ultraviolet radiation
and reactions with solid particles in the atmosphere.13
- Groundwater contamination is unlikely based on the present use pattern and volatility of sulfuryl fluoride.1
- Exposure to non-target organisms is unlikely based on the present use pattern of sulfuryl fluoride.1
- Wildlife may be exposed to low concentrations of sulfuryl fluoride for a short period of time during the ventilation process.
Adverse effects are unexpected based on mammalian inhalation toxicity data.25
- The pesticide label requires that pest control companies provide an information sheet to an adult occupant of the structure,
prior to fumigation. The pesticide fact sheet contains important information on health risks, safety precautions, and
preparations.1,6 A product label may also be available from the applicator.
- People, animals, plants, water proof covers, and items covered with plastic (plastic can slow down the aeration process)
should be removed from the structure. In addition, food, feed, and medicines that no longer have the manufacturer's airtight
seal intact should also be removed or double bagged in special bags (available from your pest control company).
Don't forget to remove items in refrigerators and freezers. Turn off all flames: e.g., pilot lights and electric heating elements.6
- As a result of sulfuryl fluoride's low water solubility, wetting the soil around the perimeter of the structure will help prevent
loss of the fumigant near the base of the tent and reduce exposure to plant roots.3,13,14 Sulfuryl fluoride is phytoxic.2,3,4
- Please check with your Pest Control Company for additional preparations that may be required.
Date Reviewed: February 2000; some updates made in 2011
Please cite as: Sulfuryl Fluoride Technical Fact Sheet; National Pesticide Information Center, Oregon State University
Extension Services, 2000. https://npic.orst.edu/factsheets/archive/sftech.html.
References:
- Reregistration Elegibility Decision Document (RED) Sulfuryl Fluoride; EPA-738-R-93-016; U.S. Environmental Protection Agency, Office
of Prevention, Pesticides and Toxic Substances, Office of Pesticide Programs, U.S. Government Printing Office: Washington, DC, 1993.
- Tomlin, C. D. S. The Pesticide Manual: A World Compendium, 11th ed.; British Crop Protection Council: Farnham, Surray, UK, 1997.
- Kenaga, E. E. Biological, chemical, and physical properties of sulfuryl fluoride as an insecticidal fumigant. J. Econ. Entomol. 1957, 50, 1-6.
- Stewart, D. Sulfuryl fluoride, new fumigant for control of the drywood termite Kalotermes minor. J. Econ. Entomol. 1957, 50, 7-11.
- Pesticide Fact Sheet Number 51: Sulfuryl Fluoride; U.S. Environmental Protection Agency, Office of Prevention, Pesticides and Toxic
Substances, Office of Pesticide Programs, U.S. Government Printing Office: Washington, DC, 1985.
- Specimen Label: Vikane Specialty Gas Fumigant; DowElanco: Indianapolis, IN, 1996.
- Bennett, G. W., Owens, J. M., Corrigan, R. M. Truman's Scientific Guide to Pest Control Operations, 5th ed; Advanstar Communications,
Inc.: Cleveland, OH, 1997; pp 447-464.
- Clarkson, T. W. Inorganic and Organometal Pesticides. Handbook of Pesticide Toxicology: Classes of Pesticides. Academic Press, Inc.: San
Diego, CA, 1991; Vol. 2.
- Meikle, R. W.; Stewart, D.; Globus, O. A. Drywood termite metabolism of Vikane fumigant as shown by labeled pool technique. J. Agric. Food Chem. 1963, 11, 226-30.
- Osbrink, W. L. A.; Scheffrahn, R. H.; Su, N. Y.; Rust, M. K. Laboratory comparisons of sulfuryl fluoride toxicity and mean time of mortality
among ten termite species (Isoptera: Hodotermitidae, Kalotermitidae, Rhinotermitidae). J. Econ. Entomol. 1987, 80, 1044-7.
- Outram, I. Effects of the fumigant sulfuryl fluoride on the gross metabolism of insect eggs. Fluoride Quart. Rep. 1970, 3, 85-91.
- Structural fumigation using sulfuryl fluoride: Dow Elanco's Vikane Gas Fumigant (Methyl Bromide alternative case study); EPA-430-R-96-021.
U.S. Environmental Protection Agency, Office of Prevention, Pesticides and Toxic Substances, Office of Pesticide Programs, U.S. Government Printing Office: Washington, DC, 1996; Vol. 2.
- General Information on Vikane Gas Fumigant; Form no. 311-56-077; DowElanco: Indianapolis, IN, 1994.
- Vikane Gas Fumigant: Structural Fumigation Manual; DowElanco: Indianapolis, IN, 1996.
- Gehring, P. J.; Nolan, R. J.; Watanabe, P. G.; Schumann, A. M. Handbook of Pesticide Toxicology: Classes of Pesticides. Academic Press, Inc.:
San Diego, CA, 1991; p 675-676, Vol. 2.
- Sulfuryl Fluoride; Pesticide Tolerance. Fed. Reg. January 23, 2004, 69 (15), 3240-3257.
- Eisenbrandt, D. L.; Nitschke, K. D. Inhalation toxicity of sulfuryl fluoride in rats and rabbits. Fundam. Appl. Toxicol. 1989, 12, 540-57.
- Nitschke, K. D.; Albee, R. R.; Mattsson, J. L.; Miller, R. R. Incapacitation and treatment of rats exposed to a lethal dose of sulfuryl
fluoride. Fundam. Appl. Toxicol. 1986, 7, 664-70.
- Reigart, J. R.; Roberts, J. R. Fumigants. Recognition and Management of Pesticide Poisonings; EPA-735-R-98-003; U.S. Environmental
Protection Agency, Office of Prevention, Pesticides and Toxic Substances, Office of Pesticide Programs, U.S. Government Printing
Office: Washington, DC, 1999.
- Fatalities resulting from sulfuryl fluoride exposure after home fumigation-Virginia. Morb. Mortal. Wkly. Rep. 1987, 36 (36), 602-4, 609-11.
- Hanley, T. R., Jr.; Calhoun, L. L.; Kociba, R. J.; Greene, J. A. The effects of inhalation exposure to sulfuryl fluoride on fetal development in
rats and rabbits. Fundam. Appl. Toxicol. 1989, 13, 79-86.
- Mühle, J.; Huang, J.; Weiss, R. F.; Prinn, R. G.; Miller, B. R.; Salameh, P. K.; Harth, C. M.; Fraser, P. J.; Porter, L. W.; Greally, B. R.; O'Doherty, S.;
Simmonds, P. G. Sulfuryl fluoride in the global atmosphere. J. Geophys. Res. 2009, 114 (D5), D05306.
- Papadimitriou, V. C.; Portmann, R. W.; Fahey, D. W.; Mühle, J.; Weiss, R. F.; Burkholder, J. B. Experimental and Theoretical Study of the
Atmospheric Chemistry and Global Warming Potential of SO2F2. J. Phys. Chem. A 2008, 112 (49), 12657-12666.
- Sulbaek Andersen, M. P.; Blake, D. R.; Rowland, F. S.; Hurley, M. D.; Wallington, T. J. Atmospheric Chemistry of Sulfuryl Fluoride: Reaction
with OH Radicals, Cl Atoms and O3, Atmospheric Lifetime, IR Spectrum, and Global Warming Potential. Environ. Sci. Technol. 2009, 43 (4), 1067-1070.
- Kamrin, M. A. Pesticide Profiles: Toxicity, Environmental Impact, and Fate; Lewis Publishers: New York, 1997.




