Laboratory Testing: Before pesticides are registered by
the U.S. EPA, they must undergo laboratory testing for
short-term (acute) and long-term (chronic) health effects.
Laboratory animals are purposely given high enough doses
to cause toxic effects. These tests help scientists judge how
these chemicals might affect humans, domestic animals,
and wildlife in cases of overexposure.
- d-Phenothrin, also referred to as phenothrin or sumithrin, is a
type I pyrethroid insecticide.1 Pyrethroids are synthetic chemicals
modeled after the pyrethrin components of pyrethrum,
which is derived from plants.2 The International Union of Pure
and Applied Chemistry (IUPAC) name for d-phenothrin is 3-phenoxybenzyl
(1RS,3RS;1RS,3SR)-2,2-dimethyl-3-(2-methylprop-1-
enyl)cyclopropanecarboxylate, and the Chemical Abstracts Service
(CAS) registry number is 26002-80-2.3
- Phenothrin was first synthesized in 1969.4 d-Phenothrin was first registered with the United States Environmental Protection
Agency (U.S. EPA) in 1976.1 See the text box on Laboratory Testing.
Molecular Structure -
d-Phenothrin
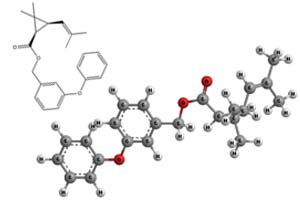
- d-Phenothrin and phenothrin are similar in chemical structure
but differ by isomeric configuration. Phenothrin is made up of
equal parts of four isomers (1R-cis, 1R-trans, 1S-cis, 1S-trans),
while d-phenothrin contains ≥95% 1R isomers, and is a 1:4 mixture
of the 1R-cis and 1R-trans isomers. The 1R-trans isomer is
more toxic than the 1R-cis isomer.3,4
- Vapor pressure1: 1.43 x 10-7 mmHg at 21 °C
- Octanol-Water Partition Coefficient (log Kow)1,3: 6.01
- Henry's constant1: 6.80 x 10-6 atm·m3/mol
- Molecular weight1: 350.46 g/mol
- Solubility (water)1,3: <9.7 μg/L at 25 °C
- Soil Sorption Coefficient (Koc)5,6: 1.25 x 105 to 1.41 x 105
- d-Phenothrin is registered for use in commercial and industrial settings, medical institutions, and other settings including
aircraft, railroad cars, ships, trailers, homes, animal kennels, gardens, greenhouses, and pet products.1,7 Uses for individual
products containing d-phenothrin vary widely. Always read and follow the label when applying pesticide products.
- d-Phenothrin is also registered for application by aircraft or truck-mounted sprayers as an ultra-low volume (ULV) application
for mosquito control in vector abatement programs.1,7
- Signal words for products containing d-phenothrin may range from Caution to Danger. The signal word reflects the combined
toxicity of the active ingredient and other ingredients in the product. See the pesticide label on the product and refer to
the NPIC fact sheets on Signal Words and Inert or "Other" Ingredients.
- To find a list of products containing d-phenothrin which are registered in your state, visit the website
https://npic.orst.edu/reg/state_agencies.html select your state then click on the link for "State Products."
Target Organisms
- d-Phenothrin kills insects by direct contact and ingestion.1,3 d-Phenothrin is a Type I pyrethroid that affects the insect's central
and peripheral nervous system.1 Following exposure to d-phenothrin, voltage-gated sodium channels are kept open
for prolonged periods of time, causing repetitive nerve discharge and increased excitation.8,9
Non-target Organisms
- d-Phenothrin and other pyrethroid insecticides are generally lower in toxicity to mammals than insects because they
interfere with sodium channels more strongly at lower temperatures. The body temperature of insects and other invertebrates
is approximately 10 degrees below that of mammals. Higher mammalian body temperatures also contribute to the
increased metabolic degradation of pyrethroids.8
- Cats may have an increased sensitivity to the effects of pyrethroid insecticides, including d-phenothrin.1,10
LD50/LC50: A common measure of acute toxicity is the lethal
dose (LD50) or lethal concentration (LC50) that causes death
(resulting from a single or limited exposure) in 50 percent
of the treated animals. LD50 is generally expressed as the
dose in milligrams (mg) of chemical per kilogram (kg) of
body weight. LC50 is often expressed as mg of chemical
per volume (e.g., liter (L)) of medium (i.e., air or water) the
organism is exposed to. Chemicals are considered highly
toxic when the LD50/LC50 is small and practically non-toxic
when the value is large. However, the LD50/LC50 does not
reflect any effects from long-term exposure (i.e., cancer,
birth defects or reproductive toxicity) that may occur at
levels below those that cause death.
Oral
- d-Phenothrin is very low in toxicity when ingested by rats, with
an acute oral LD50 >5,000 mg/kg.1 See the text boxes on Toxicity
Classification and LD50/LC50.
Dermal
- d-Phenothrin is low in toxicity to rats when applied to the skin,
with an acute dermal LD50 >2,000 mg/kg.1
- d-Phenothrin is not a skin irritant or skin sensitizer in rabbits and
guinea pigs.1
- d-Phenothrin is a mild eye irritant in rabbits.1
Inhalation
- d-Phenothrin was very low in toxicity when inhaled by rats, with a 4-hour inhalation LC50 >2.1 mg/L.1
Signs of Toxicity - Animals
- Type I pyrethroids including d-phenothrin have been associated with acute signs of neurotoxicity when administered to
rats via oral or intravenous routes of exposure. Signs of acute toxicity in animals may include aggression, increased stimulus
response, convulsive twitching, tremors, coma, and death.11
- Other signs of toxicity in animals include excessive salivation, ear twitching, and paw flicking from oral and dermal sensory
nerve stimulation. Animals may also experience vomiting, diarrhea, and topical allergic reactions.12
- Cats may be particularly sensitive to d-phenothrin. Neurotoxic symptoms including tremors, excess salivation and seizures
have been reported following treatment of cats with spot-on flea and tick products containing d-phenothrin. As a result,
flea and tick spot-on products containing d-phenothrin were canceled for use on cats and kittens in 2005.1
| TOXICITY CLASSIFICATION - d-PHENOTHRIN |
|
High Toxicity |
Moderate Toxicity |
Low Toxicity |
Very Low Toxicity |
| Acute Oral LD50 |
Up to and including 50 mg/kg
(≤ 50 mg/kg) |
Greater than 50 through 500 mg/kg
(>50-500 mg/kg) |
Greater than 500 through 5000 mg/kg
(>500-5000 mg/kg) |
Greater than 5000 mg/kg
(>5000 mg/kg) |
| Inhalation LC50 |
Up to and including 0.05 mg/L
(≤0.05 mg/L) |
Greater than 0.05 through 0.5 mg/L
(>0.05-0.5 mg/L) |
Greater than 0.5 through 2.0 mg/L
(>0.5-2.0 mg/L) |
Greater than 2.0 mg/L
(>2.0 mg/L) |
| Dermal LD50 |
Up to and including 200 mg/kg
(≤200 mg/kg) |
Greater than 200 through 2000 mg/kg
(>200-2000 mg/kg) |
Greater than 2000 through 5000 mg/kg
(>2000-5000 mg/kg) |
Greater than 5000 mg/kg
(>5000 mg/kg) |
| Primary Eye Irritation |
Corrosive (irreversible destruction of
ocular tissue) or corneal involvement or
irritation persisting for more than 21 days |
Corneal involvement or other
eye irritation clearing in 8 - 21 days |
Corneal involvement or other
eye irritation clearing in 7
days or less |
Minimal effects clearing in less than 24 hours |
| Primary Skin Irritation |
Corrosive (tissue destruction into the
dermis and/or scarring) |
Severe irritation at 72 hours
(severe erythema or edema) |
Moderate irritation at 72
hours (moderate erythema) |
Mild or slight irritation at
72 hours (no irritation or
erythema) |
| The highlighted boxes reflect the values in the "Acute Toxicity" section of this fact sheet. Modeled after the U.S. Environmental Protection Agency, Office of Pesticide Programs, Label Review Manual, Chapter 7: Precautionary Labeling. https://www.epa.gov/sites/default/files/2018-04/documents/chap-07-mar-2018.pdf |
Signs of Toxicity - Humans
- Poison Control Center (PCC) data c ollected from 1993-2005 showed that on average, 180 exposures to d-phenothrin were
reported each year. About one quarter of those cases involve reported symptoms. These included nausea, vomiting, throat
irritation, headache, dizziness, and skin and eye irritation.13
- The National Institute for Occupational Safety and Health (NIOSH) Sentinel Event Notification System for Occupational
Risks (SENSOR) reported four cases of occupational exposure to phenothrin between 1998 and 2003. The reports involved
mild symptoms, including exacerbation of nasal allergies, itching, and asthmatic responses.13
- Paresthesia is associated with exposure to pyrethroids, though more commonly with type II pyrethroids containing a cyano-
group.14 Paresthesia is characterized by sensations of itching, stinging, burning, and tingling of the skin, which may
progress to numbness. Symptoms usually occur within 1-2 hours of exposure, and do not typically last more than 24-48
hours.14,15
- In an analysis of worker exposure data, the most common symptom reported for individuals working with pyrethroids was
paresthesia following dermal contact. Paresthesia was generally limited to the direct point of contact with the skin, and
occurred without signs of skin irritation. Paresthesia effects generally resolved within 48 hours of exposure, and no clinical
signs of systemic toxicity were observed.15
- Systemic toxicity through skin contact and inhalation of pyrethroids is low. Effects to the central nervous system (e.g. seizures)
are rare, but have been reported in cases of severe exposure. Seizures are more common with exposure to type II
pyrethroids.14
- Always follow label instructions and take steps to minimize exposure. If any exposure occurs, be sure to follow the First Aid
instructions on the product label carefully. For additional treatment advice, contact the Poison Control Center at 1-800-
222-1222. If you wish to discuss an incident with the National Pesticide Information Center, please call 1-800-858-7378.
Animals
NOAEL: No Observable Adverse Effect Level
NOEL: No Observed Effect Level
LOAEL: Lowest Observable Adverse Effect Level
LOEL: Lowest Observed Effect Level
- Researchers fed phenothrin to dogs at doses of 0, 100, 300, 1000, or
3000 ppm for 52 weeks. At the highest dose researchers observed
anemia, decreased serum proteins, and increased liver weights in
both sexes. The LOAEL was 1000 ppm (26.8 mg/kg/day) based on enlargement
of liver cells and focal degeneration in the adrenal cortex
in male and female dogs. The systemic NOAEL was 300 ppm (7.1 mg/
kg/day).7,16 See the text box on NOAEL, NOEL, LOAEL, and LOEL.
- Researchers exposed male and female rats to d-phenothrin via inhalation at concentrations of 0, 0.030, 0.104, 0.291, or
1.066 mg/L, 6 hours/day, 5 days/week, for 13 weeks and observed no adverse effects at 0.30 or 0.104 mg/L. The LOAEL was
0.291 mg/L based on changes in nasal tissue in male and female rats. Changes were also observed in the thyroid, liver, and
adrenals at 1.066 mg/L.17
- Scientists applied phenothrin to the skin of rats at doses of 0, 100, 300, or 1000 mg/kg/day for 21 days. No systemic effects
were observed.18
Humans
- No human data were found on chronic effects of d-phenothrin.
- Endocrine-related effects in dogs that were fed 1000 ppm d-phenothrin included liver cell enlargement after 6 months and
vacuole formation in the adrenal cortex after 1 year.7,16
- Researchers performed a 90-day inhalation study in rats.7 Endocrine-related effects included minor vacuole formation in
the adrenal cortex and follicular thyroid cell enlargement in males at 0.291 and 1.066 mg/L, and liver cell enlargement at
1.066 mg/L.7,17
- Researchers conducted separate studies on female and male rats to determine if d-phenothrin exhibits adverse hormone
activity. In the first study, immature female rats were fed d-phenothrin for 3 days at doses of 0, 100, 300, or 1000 mg/kg/day.
Researchers found no estrogenic effects at any dose. In the second study, scientists fed d-phenothrin to castrated peripubertal
male rats for 10 days at the same doses and found no androgenic/antiandrogenic effects.19
Animals
- d-Phenothrin is classified by the U.S. EPA as "not likely to be carcinogenic to humans" based on liver tumors and growths
being observed only at excessively toxic doses (20,000 ppm) in rats, and the lack of statistical significance of these findings.7
- Researchers fed d-phenothrin to mice at doses of 0, 300, 1000, or 3000 ppm for 2 years. They observed no evidence of carcinogenicity.20
- Researchers often use studies designed to test for mutagenicity to screen chemicals for carcinogenicity. The U.S. EPA concluded
that d-phenothrin "does not pose a mutagenic concern" based on negative findings in bacterial and mammalian
gene mutation studies, and a chromosomal aberration study using mammalian cells.7 See the text box on Cancer.
Cancer: Government agencies in the United States and abroad have developed programs to evaluate the
potential for a chemical to cause cancer. Testing guidelines and classification systems vary. To learn more
about the meaning of various cancer classification descriptors listed in this fact sheet, please visit the
appropriate reference, or call NPIC.
Humans
- No human data were found on carcinogenic effects of d-phenothrin.
Animals
- In a two-generation reproductive study, researchers fed phenothrin to rats (initial and first generation offspring following
weaning) in the diet at doses of 0, 300, 1000, or 3000 ppm for 13 weeks. The first generation offspring mated to produce a
second generation. At doses below 3000 ppm, no systemic toxicity was observed in the parental generation. At 3000 ppm
(177.2 mg/kg/day), researchers observed decreased food consumption, decreased body weight, increased liver weights
and cellular changes in the liver. At the same dose, the offspring had lower body weights during lactation. The reproductive/
offspring LOAEL is 3000 ppm (177.2 mg/kg/day) based on decreased body weight of offspring during lactation.21
- Researchers gave phenothrin to pregnant rabbits by gavage during days 7-19 of gestation at doses of 0, 30, 100, 300, or 500
mg/kg/day. They observed signs of maternal toxicity, including decreased food consumption and weight gain at 300 and
500 mg/kg/day, and decreased elimination and increased abortions at 500 mg/kg/day.22 The developmental NOAEL is 30
mg/kg/day based on spina bifida in one fetus at 100 mg/kg/day.23
- Scientists fed phenothrin to pregnant rabbits at doses of 0, 3, 10, or 30 mg/kg/day during days 6-18 of gestation and observed
no birth defects in the offspring.24
Humans
- No human data were found on the teratogenic or reproductive effects of d-phenothrin.
Absorption
- Pyrethroids are rapidly absorbed by the gastrointestinal tract following ingestion.15
- Pyrethroids are likely to be efficiently absorbed from the respiratory tract following inhalation.15
- Pyrethroids are poorly absorbed through the skin following dermal exposure. Researchers applied phenothrin isomers to
the skin of rats as a dust or as an emulsifiable concentrate (EC) at 0.2 mg/rat and 2 mg/rat for a duration of 24 hours. After
6 days, 78-85% of the EC and 86-94% of the dust was recovered from the skin, unabsorbed.25
- Phenothrin isomers absorbed more quickly across the skin when applied as an emulsifiable concentrate compared to a
dust formulation.25
Distribution
- Pyrethroids, including d-phenothrin, are lipophilic. Following absorption from inhalation or ingestion, the chemical is distributed
throughout the body primarily to fatty tissues.15
- Researchers administered a single oral dose of 200 mg/kg d-phenothrin to rats and it was rapidly absorbed in the gastrointestinal
tract and distributed to body tissues and organs. Peak concentrations occurred at three hours after dosing, and
occurred in the liver, kidneys, and blood.26
- Following a single oral dose of d-phenothrin in rats (10 mg/kg), researchers observed low overall residue levels of 1.0-2.5
mg/kg in tissues after seven days, with the highest concentrations in the fat.4
Metabolism
- In mammals, d-phenothrin is primarily degraded by hydrolysis at the ester bond, oxidation of the subsequent alcohol, followed
by conjugation reactions.4,26
- Metabolites of d-phenothrin detected in the brain, liver, kidneys, and blood of rats following oral administration include
3-phenoxybenzyl alcohol, 3-phenoxybenzoic acid, and 3-(4'-hydroxy) phenoxybenzoic acid in descending order.26
- Following d-phenothrin administration in rats, researchers found the major urinary metabolites were cleaved esters, primarily
4'OH-phenoxybenzoic acid sulfate.7
Excretion
- Scientists administered a single oral dose of radiolabeled d-trans-phenothrin to rats at 200 mg/kg and found that >95% of
the compound was metabolized and excreted within 24 hours, with the remaining amounts completely recovered within
48 hours.26 The rats excreted approximately 57% of the administered dose in the urine, and approximately 44% in the feces.26
- Researchers administered a single oral dose of phenothrin to rats. Approximately 96% was recovered in the feces and urine
within 6 days. Excretion of the cis-isomer occurred at 74% in the feces and 22% in the urine, and at 75% in the urine and
21% in the feces for the trans-isomer.25
- Excessive accumulation and persistence of pyrethroids, including d-phenothrin, in the body is not expected because they
are rapidly metabolized under normal conditions.15
- Analytical methods have been developed to detect d-phenothrin metabolites in the urine using gas chromatography and
mass spectrometry.27 Detection of urinary metabolites serves as an indicator of exposure, but are insufficient to determine
the level of exposure or whether an adverse effect will occur.28
- Pyrethroids break down rapidly in the body. Therefore, analytical methods are pertinent only when exposure has occurred
within the last few days.28 Most clinical laboratories do not offer testing services for pyrethroids due to the specialized
equipment that is necessary to analyze samples.28
The "half-life" is the time required for half of the
compound to break down in the environment.
1 half-life = 50% remaining
2 half-lives = 25% remaining
3 half-lives = 12% remaining
4 half-lives = 6% remaining
5 half-lives = 3% remaining
Half-lives can vary widely based on environmental
factors. The amount of chemical remaining after a
half-life will always depend on the amount of the
chemical originally applied. It should be noted that
some chemicals may degrade into compounds of
toxicological significance.
Soil
- d-Phenothrin is degraded in the environment primarily through UV
light reactions.29
- In upland conditions, the half-life of d-phenothrin in the soil is 1-2 days.
In flood conditions, the half-life of d-phenothrin in the soil ranged
from 2 weeks up to 2 months.5,30 See the text box on Half-life.
- d-Phenothrin has low water solubility and binds tightly to soil. Based
on these properties, d-phenothrin is relatively immobile in soil and its
potential to contaminate groundwater is low.5
Water
- d-Phenothrin is not likely to contaminate groundwater due to its low water solubility and high affinity for binding to soil.5
- Under anaerobic conditions, d-phenothrin is stable to hydrolysis at all pH values with an anaerobic aquatic half-life of 173
days.5,31
- With exposure to sunlight, such as in clear shallow water, d-Phenothrin is readily degraded through aqueous photolysis
with a half-life of 6.5 days.32 However, d-phenothrin is lipophilic and has a high affinity to bind to suspended solids and
bottom sentiment in water so it is only available for photolysis for a short time.5
- d-Phenothrin has a high potential to reach surface water through erosive runoff events due to its low water solubility and
a high affinity to bind to soil.5
Air
- d-Phenothrin is readily degraded in the air through photolytic reactions with ozone and atmospheric hydroxyl radicals,
with an estimated atmospheric half-life of 38.4 minutes and 1.2 hours, respectively.5
Plants
- Researchers applied phenothrin isomers to the leaves of kidney beans and rice plants grown in greenhouses, and determined
a foliar half-life of less than 1 day.30
- Phenothrin isomers were partially taken up by the roots of bean plants grown in treated soil at concentrations of 1 ppm.
Researchers observed very little translocation into untreated parts of the plants, including the shoots and edible portions
(pods and seeds) 30 days after planting.30
- Though no phytotoxicity data were available, the U.S. EPA does not expect d-phenothrin to be toxic to plants.5
Indoor
- Scientists sprayed the corners of a room with an aerosol product containing 0.9 g d-phenothrin and 1.1 g tetramethrin in
300 mL four times over an 8-week period. Air concentrations of d-phenothrin peaked immediately after spraying (752 μg/
m3) then decreased rapidly. With minimum air exchange, the half-life of d-phenothrin in the air was 20 minutes. d-Phenothrin
did not accumulate in indoor air with repeat applications.33
- In the same study, d-phenothrin residues on the floor, ceiling, and walls gradually increased with the number of sprayings
during the 8-week period. Residue levels decreased over time when spraying was discontinued. The half-life of d-phenothrin
was 13-20 days on the floor and 31-41 days on the walls, and 24-75 days on the ceiling.33
Food Residue
- d-Phenothrin has a 0.01 ppm food tolerance for all food/feed crops following area-wide mosquito applications.34
- In 2009, the United States Department of Agriculture (USDA) tested almost 9,000 agricultural samples for d-phenothrin
residue. d-Phenothrin was not detected in any of these samples.35
- The United States Food and Drug Administration (FDA) Pesticide Residue Monitoring Program conducts regulatory and
incidence/level monitoring for pesticide residues in domestic and imported foods (except meat, poultry, dairy, and eggs).
From 2000-2008, the FDA analyzed more than 700 domestic and 1200 imported food samples for phenothrin residue. Of
the samples tested, only one sample had detectable residue at 0.022 ppm.36
Birds
- d-Phenothrin is practically non-toxic to birds. The acute oral LD50 in bobwhite quail (Colinus virginianus) is >2,510 mg/kg and the dietary LC50 is
>5,000 mg/kg.37
- No studies evaluating the chronic toxicity of d-phenothrin in birds were found. However, the U.S. EPA concluded that the
chronic avian risk from d-phenothrin is likely to be low based on the lack of chronic risk in mammalian species and the current
use pattern of d-phenothrin.1
Fish and Aquatic Life
- d-Phenothrin is very highly toxic to freshwater fish. The 96-hour LC50 is 16.7 μg/L and 15.8 μg/L in rainbow trout (Oncorhynchus mykiss) and bluegill
sunfish (Lepomis macrochirus), respectively.38
- Scientists exposed freshwater fish (rainbow trout) to d-phenothrin. Post-hatch 60-day survival in rainbow trout decreased
at doses above 1.1 μg/L.39
- d-Phenothrin is very highly toxic to estuarine/marine fish. In separate studies, the 96-hour LC50 was >38.3 μg/L and 94.3
μg/L in inland silverside (Menidia beryllina).40,41
- d-Phenothrin is very highly toxic to freshwater invertebrates. The 48-hour LC50 is 4.4 μg/L in Daphnia magna.42
- The chronic NOAEC for freshwater invertebrates is 0.47 μg/L based on a 21-day reproduction endpoint in Daphnia
magna.43
- Researchers conducted a 96-hour static toxicity study to determine the toxicity of technical and synergized (piperonyl
butoxide) formulations of d-phenothrin in crayfish. Researchers found d-phenothrin to be highly toxic to test species Orconectes
immunis (small and large) and Procambarus clarkii (large) with a LOAEC of 0.08 μg/L in tests with synergized dphenothrin
in Orconectes immunis (small). For technical and synergized d-phenothrin formulations 96-hour lethal concentrations
ranged from 0.24 μg/L in Orconectes immunis (small) to 2.62 μg/L in Orconectes immunis (large). Researchers did
not find a clear pattern of the effects of synergized formulations of d-phenothrin.44
- d-Phenothrin is very highly toxic to estuarine/marine invertebrates. The 96-hour LC50 is 0.025 μg/L in mysid shrimp (Mysidopsis bahia).40
Terrestrial Invertebrates
- d-Phenothrin is highly toxic to honey bees. The contact LD50 is 0.067 μg/bee.5
Reference Dose (RfD): The RfD is an estimate of the quantity of
chemical that a person could be exposed to every day for the rest
of their life with no appreciable risk of adverse health effects. The
reference dose is typically measured in milligrams (mg) of chemical
per kilogram (kg) of body weight per day.
U.S. Environmental Protection Agency, Integrated Risk Information System, IRIS Glossary, 2009. https://www.epa.gov/iris/iris-glossary#r
- The acute Reference Dose (aRfD) for 13-49 year old
females is 0.03 mg/kg based on a developmental NOAEL
of 30 mg/kg/day in rabbits.7,23 See the text box on Reference Dose (RfD).
- The chronic RfD for d-phenothrin is 0.007 mg/kg/day
based on a systemic toxicity NOAEL of 7.1 mg/kg/day in dogs.7
- d-Phenothrin is classified as "not likely to be carcinogenic to humans".7 See the text box on Cancer.
- The Acceptable Daily Intake (ADI) for d-phenothrin is 0.07 mg/kg.3
Date Reviewed: September 2011
Please cite as: Jackson, D.; Luukinen, B.; Gervais, J.; Buhl, K.; Stone, D. 2011. d-Phenothrin Technical Fact Sheet; National
Pesticide Information Center, Oregon State University Extension Services. https://npic.orst.edu/factsheets/archive/dphentech.html.



